Research
New COVID-19 antigen testing method offers highly accurate results in under 3 minutes
September 30, 2021
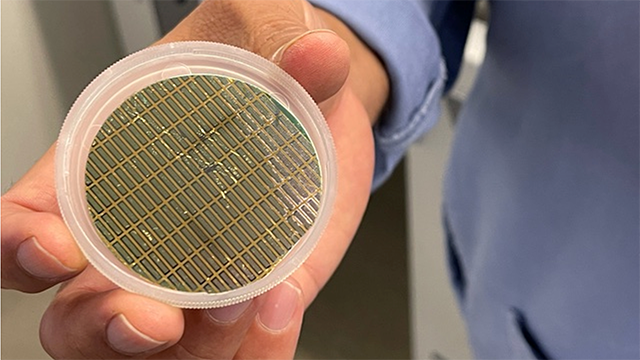
The platform uses microscopic cantilevers.
Rapid antigen nasal swab test eliminated false positives and detected COVID-19 spike proteins before the onset of symptoms
New variants to the COVID-19 virus have challenged the capacity of existing testing methods to deliver fast and consistently accurate test results, as PCR tests must be sent to labs and can take days to be returned. Furthermore, rapid antigen alternatives struggle to match up to the gold standard. But to effectively slow the rate of infection, spaces like schools and hospitals need ways to accurately identify infected individuals within their first days of infection as they’re walking in the door.
Now, a novel antigen-based COVID-19 detection method developed in a Northwestern University laboratory demonstrated 100% accuracy in a blind test in five or fewer minutes from swab to signal.
The rapid swab test uses a nanomechanical platform to detect multiple surface proteins on the COVID-19 virus, and shows potential to differentiate among different variants and viruses. The high-sensitivity test is also being developed as a rapid exhaled breath COVID-19 detection method, similar to a breathalyzer test.
Published in the journal Biosensors and Bioelectronics, the paper is titled “Highly sensitive and ultra-rapid antigen-based detection of SARS-CoV-2 using nanomechanical sensor platform.” A pre-proof version of the paper is available online. The final version will be published tomorrow (Oct. 1).
The platform, which leverages microscopic cantilevers, was originally developed 15 years ago to detect protein interactions with DNA, so could effectively be “dusted off” and repurposed, according to corresponding author and Northwestern materials science and engineering professor Vinayak P. Dravid.
“We were excited to apply the technology because these are micro- and nanosystems that don’t need a lot of viral material to make a difference,” Dravid said. “Microcantilevers can give you a faster turnaround — within two or three minutes — because they leverage specific affinity surface binding. And unlike most sensors available that rely on just one protein, we can look at multiple targets at the same time.”
The tiny cantilevers are made of silicon and can be easily reproduced by following a mold, making them an attractive option for potential mass-production. After optimizing the old technology for use with electronic imaging, Dravid’s lab worked with scientists at the Northwestern University Feinberg School of Medicine to acquire patient swab samples and use data to understand how diseases move through communities.
The team applied coatings of different COVID-19 antigens onto each “finger” of five silicon cantilevers next to an additional one for reference. Then, collected swab samples were grafted onto the cantilever surface. If COVID-19 antibodies were present in the sample, they caused the thin cantilever with corresponding antigens to bend, with each finger serving as an extra line of defense for any other antigens.
Gajendra S. Shekhawat, a co-corresponding author and Northwestern materials science and engineering research professor, said the technology is not limited to COVID-19 detection.
“We have some initial data to demonstrate the high sensitivity to other diseases in addition to the coronavirus,” Shekhawat said. “We also are developing integrated microfluidics that will allow us to dip multiple cantilevers into antibody solutions and detect multiple viral loads at the same time.”
In addition to incorporating multiple variants and diseases into the mechanism, the team’s goal is to expand the platform to hold more capabilities. Dravid said they are looking for ways to scale and produce the technology and ultimately bring it to mobile devices, using the approach to bring no-contact testing to the public.
“Instead of nasal swabs, we want to use breath,” Dravid said. “Because the sensitivity of the technique is so good, breath has a lower viral load, but it has enough virus for this technology to detect.”
Shekhawat said children back in school combined with the well-researched fact that underserved populations are at higher risk of exposure underscores the need for non-invasive and rapid diagnostics approaches. Breath sampling could be used for managing and mitigation in future public health challenges, he said.
Dravid said he imagines people entering hotels, hospitals and schools, picking up one of his tests to breathe into, and then knowing whether they’re infected by the time they’re on the other side of the room. Developing this fast understanding of people who are sick even without displaying symptoms, Dravid said, “changes the way mitigation strategies work.”
Dravid is the Abraham Harris Professor of Materials Science and Engineering at Northwestern’s McCormick School of Engineering, the founding director of the Northwestern University Atomic and Nanoscale Characterization Experimental Center (NUANCE), and director of the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource. Dravid also serves as the director of global initiatives for Northwestern’s International Institute of Nanotechnology.
Shekhawat serves as the manager of SPID Facility of the NUANCE Center and is part of the leadership team of the SHyNE Resource.
Dilip Kumar Agarwal, a postdoctoral research associate in Dravid’s group, was the lead author of the paper. The research was supported by a grant from the National Heart, Lung and Blood Institute (Award number 1400-SUB/2U54HL119810-07S1: Rapid Diagnostics of SARS-C0V-2 of Asymptomatic people returning to work and school). The work made use of the SPID facilities of the NUANCE Center at Northwestern University which received support from Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205), MRSEC program (NSF DMR-1121262) at the Materials Research Center; the International Institute for Nanotechnology (IIN); the Keck Foundation; and the State of Illinois, through the IIN.
Sample collection was supported by a COVID-19 pilot grant from the Northwestern University Clinical and Translational Science Institute (NUCATS) (NIH grant number UL1 TR001422); a Dixon Translational Research Grant made possible by the generous support of the Dixon Family Foundation; a CTSA supplement to NUCATS (award number UL1 TR002389); and a supplement to the Northwestern University Cancer Center (award number P30 CA060553).