Nanotechnology at Northwestern, News
Breakthrough in Targeting ‘Undruggable’ Proteins Promises New Horizon for Neurodegenerative Disease Treatment
February 16, 2024
International Institute for Nanotechnology at Northwestern University Unveils Revolutionary Proteomimetic Polymer Technology in Advanced Materials
Evanston, IL – Professors Nathan Gianneschi of the International Institute for Nanotechnology at Northwestern University in collaboration with Jeffrey A. Johnson and Delinda A. Johnson, senior scientist, of the University of Wisconsin–Madison School of Pharmacy, unveil groundbreaking research with the potential to alter the treatment landscape for neurodegenerative diseases (NDs). The study, published in the esteemed journal Advanced Materials, titled “Inhibiting the Keap1/Nrf2 Protein-Protein Interaction with Protein-Like Polymers,” introduces a pioneering approach aimed at combating conditions such as Alzheimer’s disease, Parkinson’s disease (PD), and Amyotrophic lateral sclerosis (ALS).
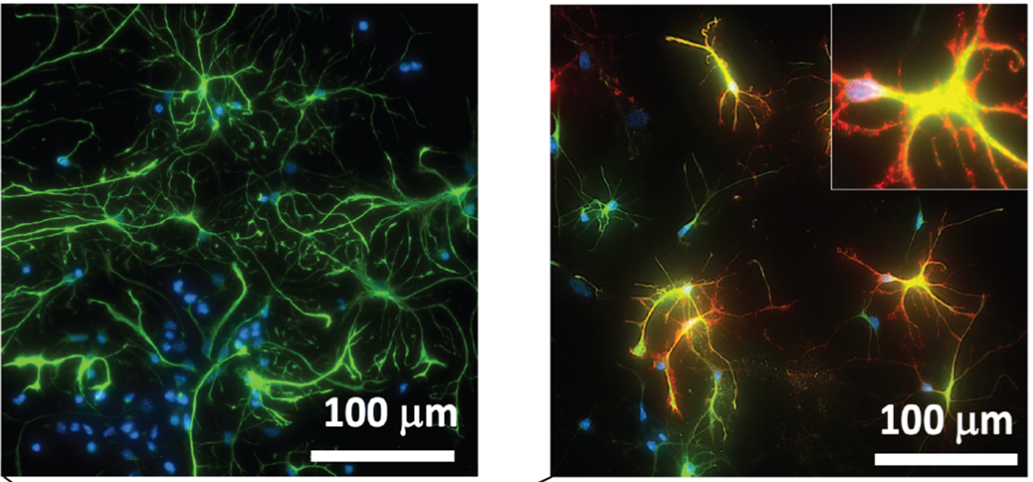
NDs, characterized by the progressive loss of neurons and glial cell dysfunction, pose a growing threat to the aging population, with Alzheimer’s alone affecting approximately 10.7% of Americans over 65 years of age. This study addresses the critical challenge of oxidative stress in NDs by targeting the Nrf2 pathway, a key regulator of cellular redox homeostasis, whose dysfunction is implicated in the pathology of these conditions.
Targeting Neurodegenerative Diseases
Alzheimer’s disease, characterized by the accumulation of beta-amyloid plaques and tau protein tangles; Parkinson’s disease, known for its loss of dopaminergic neurons and presence of Lewy bodies; and ALS, involving the degeneration of motor neurons, all share a common thread of oxidative stress contributing to disease pathology.
The study focuses on disrupting the Keap1/Nrf2 protein-protein interaction (PPI), which opens new avenues for treating neurodegenerative diseases by enhancing the body’s antioxidant response, crucial for cellular protection against oxidative stress. By preventing the degradation of Nrf2 through selective inhibition of its interaction with Keap1, the research holds promise for mitigating the cellular damage that underlies these debilitating conditions.
“We established Nrf2 as a principal target for the treatment of NDs over the past two decades, but this novel approach for activating the pathway holds great promise to develop disease-modifying therapies,” said UW–Madison Professor Jeffrey Johnson.
Limitations of Current Therapeutics
The research team embarked on addressing one of the most challenging aspects of ND treatment: the precise targeting of PPIs within the cell. Traditional methods, including small molecule inhibitors and peptide-based therapies, have fallen short due to a lack of specificity, stability, and cellular uptake.
The study introduces an innovative solution: protein-like polymers, or PLPs, are high-density brush macromolecular architectures synthesized via the ring-opening metathesis polymerization (ROMP) of norbornenyl-peptide-based monomers. These globular, proteomimetic structures display bioactive peptide side chains that can penetrate cell membranes, exhibit remarkable stability, and resist proteolysis.
This targeted approach to inhibit the Keap1/Nrf2 PPI represents a significant leap forward. By preventing Keap1 from marking Nrf2 for degradation, Nrf2 accumulates in the nucleus, activating the Antioxidant Response Element (ARE) and driving the expression of detoxifying and antioxidant genes. This mechanism effectively enhances the cellular antioxidant response, providing a potent therapeutic strategy against the oxidative stress implicated in many forms of ND.
The Innovation Behind Protein-Like Polymers
PLPs, developed by Professor Gianneschi’s team, could represent a significant breakthrough in halting or reversing damage, offering hope for improved treatments and outcomes.
Focusing on the challenge of activating processes crucial for the body’s antioxidant response, the team’s research offers a novel solution. The team provides a robust, selective method enabling enhanced cellular protection and offering a promising therapeutic strategy for a range of diseases, including neurodegenerative conditions.
“Through modern polymer chemistry, we can begin to think about mimicking complex proteins. The promise lies in the development of a new modality for the design of therapeutics. This could be a way to address diseases like Alzheimer’s and Parkinson’s among others where traditional approaches have struggled,” said Professor Nathan Gianneschi.
A Game-Changing Approach to Chemical Biology
This approach not only represents a significant advance in targeting transcription factors and disordered proteins but also showcases the PLP technology’s versatility and potential to revolutionize the development of therapeutics. The technology’s modularity and efficacy in inhibiting the Keap1/Nrf2 interaction underscore its potential for impact as a therapeutic but also as a tool for studying the biochemistry of these processes.
A Collaboration of Minds
Highlighting the study’s collaborative nature, Professor Gianneschi’s team worked closely with experts across disciplines, illustrating the rich potential of combining materials science with cellular biology to tackle complex medical challenges.
“We were contacted by Professor Gianneschi and colleagues proposing to use this novel PLP technology in NDs due to our previous work on Nrf2 in models of AD, PD, ALS, and Huntington’s Disease. We had never heard of this approach for Nrf2 activation and immediately agreed to initiate this collaborative effort that led to the generation of great data and this publication,” said Johnson, professor of pharmaceutical sciences at the UW–Madison School of Pharmacy.
This partnership underscores the importance of interdisciplinary research in developing new therapeutic modalities.
Impact
With the development of this innovative technology, Professor Nathan Gianneschi, his colleagues at the International Institute for Nanotechnology and the Johnson Lab at the University of Wisconsin–Madison, are not just advancing the field of medicinal chemistry; they are opening new pathways to combat some of the most challenging and devastating NDs faced by society today. As this research progresses towards clinical application, it may soon offer new hope to those suffering from diseases of oxidative stress, such as Alzheimer’s and Parkinson’s disease.
“By controlling materials at the scale of single nanometers, we’re opening new possibilities in the fight against diseases that are more prevalent than ever, yet remain untreatable,” says Professor Gianneschi. “This study is just the beginning. We’re excited about the possibilities as we continue to explore and expand the development of macromolecular drugs, capable of mimicking some of the aspects of proteins using our PLP platform.”
Gianneschi, along with the Johnsons, are the corresponding authors of “Inhibiting the Keap1/Nrf2 Protein-Protein Interaction with Protein-Like Polymers,” which will be published in Advanced Materials on February 16.
About the International Institute for Nanotechnology
Founded in 2000 as an umbrella organization to coalesce and foster nanotechnology efforts, the International Institute for Nanotechnology, directed by Prof. Chad A. Mirkin, represents and unites more than $1.2 billion in nanotechnology research, educational programs, and supporting infrastructure.


