Efficient Capture of Perrhenate and Pertechnetate by a Mesoporous Zr Metal–Organic Framework and Examination of Anion Binding Motifs
Chem. Mater. 2018, 30 (4), 1277-1284
 At the Hanford Site in southeastern Washington state, the U.S. Department of Energy intends to treat 56 million gallons of legacy nuclear waste by encasing it in borosilicate glass via vitrification. This process ineffectively captures radioactive pertechnetate (TcO4–) because of the ion’s volatility, thereby requiring a different remediation method for this long-lived (t1/2 = 2.1 × 105 years), environmentally mobile species. Currently available sorbents lack the desired combination of high uptake capacity, fast kinetics, and selectivity. Here, we evaluate the ability of the chemically and thermally robust Zr6-based metal–organic framework (MOF), NU-1000, to capture perrhenate (ReO4–), a pertechnetate simulant, and pertechnetate.
At the Hanford Site in southeastern Washington state, the U.S. Department of Energy intends to treat 56 million gallons of legacy nuclear waste by encasing it in borosilicate glass via vitrification. This process ineffectively captures radioactive pertechnetate (TcO4–) because of the ion’s volatility, thereby requiring a different remediation method for this long-lived (t1/2 = 2.1 × 105 years), environmentally mobile species. Currently available sorbents lack the desired combination of high uptake capacity, fast kinetics, and selectivity. Here, we evaluate the ability of the chemically and thermally robust Zr6-based metal–organic framework (MOF), NU-1000, to capture perrhenate (ReO4–), a pertechnetate simulant, and pertechnetate.
Our material exhibits an excellent perrhenate uptake capacity of 210 mg/g, reaches saturation within 5 min, and maintains perrhenate uptake in the presence of competing anions. Additionally, experiments with pertechnetate confirm perrhenate is a suitable surrogate. Single-crystal X-ray diffraction indicates both chelating and nonchelating perrhenate binding motifs are present in both the small pore and the mesopore of NU-1000. Post adsorption diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) further elucidates the uptake mechanism and powder X-ray diffraction (PXRD) and Brunauer–Emmett–Teller (BET) surface area analysis confirm the retention of crystallinity and porosity of NU-1000 throughout adsorption.
Zirconium Metal–Organic Frameworks for Organic Pollutant Adsorption
- Metal–organic frameworks (MOFs) are a class of porous, crystalline materials that have been explored as sorbents to extract organic pollutants, such as agrochemicals, dyes, and pharmaceuticals, from water.
- By capitalizing on the modular nature of MOFs, chemical functionality can be precisely tuned allowing the systematic investigation of favorable interactions.
- On deciphering structure–property relationships regarding the adsorption of organic pollutants in MOFs, design rules will guide the rational preparation of next-generation sorbents.
- Mild and nonconventional routes of MOF synthesis reduce energy demands and solvent use.
- Implementation of MOF technology is being propelled by efforts to understand and improve MOF processability.
The rapid expansion of manufacturing and the industrialization of agriculture during the 20th century pervaded surface and groundwater sources with organic contaminants including agrochemicals, dyes, and pharmaceuticals. Efficient purification of these water sources is critical to safeguard human health and Earth’s ecosystems. Of the numerous strategies investigated for water purification, adsorption has received the most attention; however, the ability to design a sorbent with high uptake capacity and selectivity for a single pollutant continues to elude researchers.
The precise synthetic control over chemical functionality offered by metal–organic frameworks (MOFs) make them ideal scaffolds for the systematic investigation of selectivity-enhancing binding interactions. Herein, we review the recent reports on the use of water-stable zirconium-based MOFs (Zr-MOFs) to extract organic pollutants from water and briefly discuss the field’s future directions.
Extraction of Inorganic Selenium from Water by a Zr Metal-Organic Framework: Investigation of Volumetric Uptake Capacity and Binding Motifs
CrystEngComm 2018, 20 (40), 6140.
 Strict monitoring and control of selenium concentrations in freshwater supplies is critical to safeguarding human health and aquatic life. A handful of previously investigated sorbents exhibit noteworthy gravimetric (mg g−1) Se uptake capacities; however, often display insufficient volumetric (mg cm−3) capacities, thereby requiring large volumes of material for commercial implementation. In pursuit of mitigating this material inefficiency, we investigated the selenite (SeO32−) and selenate (SeO42−) affinity of MOF-808, a Zr-based metal–organic framework with a high density of potential Se oxyanion binding sites.
Strict monitoring and control of selenium concentrations in freshwater supplies is critical to safeguarding human health and aquatic life. A handful of previously investigated sorbents exhibit noteworthy gravimetric (mg g−1) Se uptake capacities; however, often display insufficient volumetric (mg cm−3) capacities, thereby requiring large volumes of material for commercial implementation. In pursuit of mitigating this material inefficiency, we investigated the selenite (SeO32−) and selenate (SeO42−) affinity of MOF-808, a Zr-based metal–organic framework with a high density of potential Se oxyanion binding sites.
MOF-808 recorded exceptional volumetric and gravimetric Se oxyanion capacities of 133 mg g−1 (127 mg cm−3) and 118 mg g−1 (112 mg cm−3) for aqueous selenite and selenate, respectively. Single-crystal X-ray diffraction studies revealed that selenite and selenate can bind at the MOF node via two distinct binding motifs, an η2μ2 motif in which the oxyanion coordinates to two different metal atoms in a single node, and a μ2 motif in which the oxyanion interacts with only a single metal atom. Furthermore, powder X-ray diffraction (PXRD) patterns and N2 adsorption/desorption isotherms confirm the retention of bulk crystallinity and porosity after the uptake of Se oxyanions.
Integration of Metal–Organic Frameworks on Protective Layers for Destruction of Nerve Agents under Relevant Conditions
J. Am. Chem. Soc. 2019, 141 (51) 20016–20021.
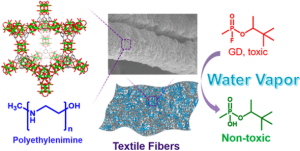 Metal–organic frameworks (MOFs) are promising candidates for the catalytic hydrolysis of nerve agents and their simulants. Though highly efficient, bulk water and volatile bases are often required for hydrolysis with these MOF catalysts, preventing real-world implementation.
Metal–organic frameworks (MOFs) are promising candidates for the catalytic hydrolysis of nerve agents and their simulants. Though highly efficient, bulk water and volatile bases are often required for hydrolysis with these MOF catalysts, preventing real-world implementation.
Herein we report a generalizable and scalable approach for integrating MOFs and non-volatile polymeric bases onto textile fibers for nerve agent hydrolysis. Notably, the composite material showed similar reactivity under ambient conditions compared to the powder material in aqueous alkaline solution. This represents a critical step toward a unified strategy for nerve agent hydrolysis in practical settings, which can significantly reduce the dimensions of filters and increase the efficiency of protective suits.
This work was highlighted in:
